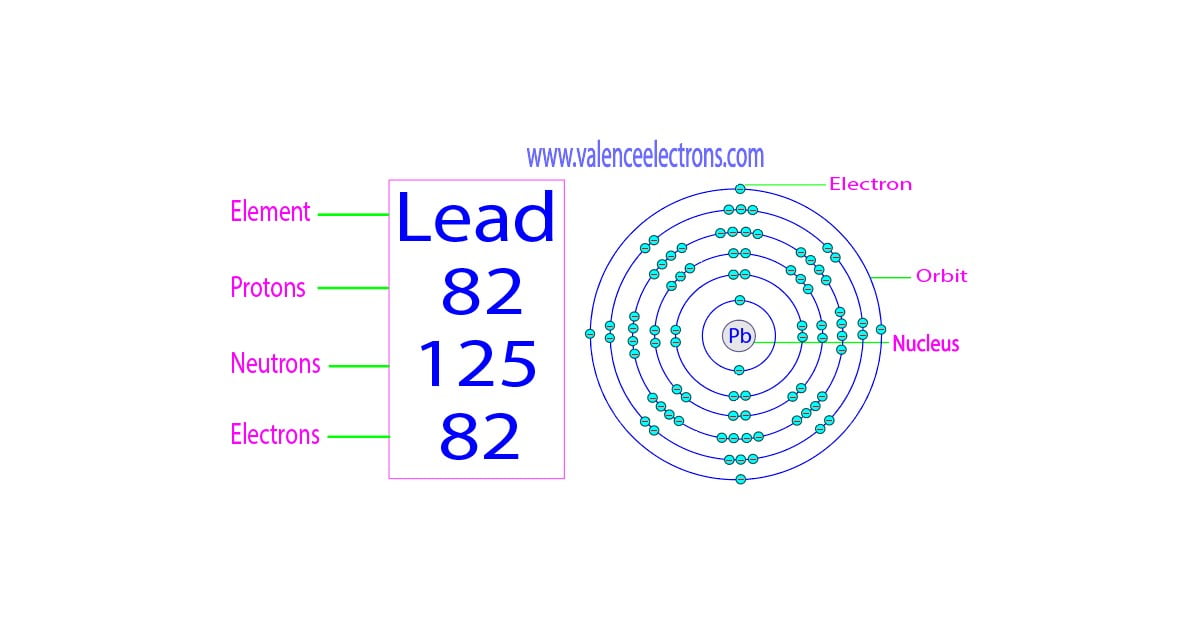
Lead-208 Electrons . 208 pb is the most common isotope, having a natural abundance of approximately 52%. The uranium series (or radium. 204 pb, 206 pb, 207 pb and 208 pb. lead occurs in 4 natural isotopes: the number of electrons in each of lead's shells is [2, 8, 18, 32, 18, 4] and its electron configuration is [xe] 4f 14 5d 10 6s 2 6p 2.
from cabinet.matttroy.net
204 pb, 206 pb, 207 pb and 208 pb. 208 pb is the most common isotope, having a natural abundance of approximately 52%. lead occurs in 4 natural isotopes: the number of electrons in each of lead's shells is [2, 8, 18, 32, 18, 4] and its electron configuration is [xe] 4f 14 5d 10 6s 2 6p 2. The uranium series (or radium.
Lead Periodic Table Protons Neutrons And Electrons Matttroy
Lead-208 Electrons 204 pb, 206 pb, 207 pb and 208 pb. 208 pb is the most common isotope, having a natural abundance of approximately 52%. The uranium series (or radium. the number of electrons in each of lead's shells is [2, 8, 18, 32, 18, 4] and its electron configuration is [xe] 4f 14 5d 10 6s 2 6p 2. lead occurs in 4 natural isotopes: 204 pb, 206 pb, 207 pb and 208 pb.
From slideplayer.com
Nomenclature and Parts of the atom ppt download Lead-208 Electrons 208 pb is the most common isotope, having a natural abundance of approximately 52%. The uranium series (or radium. lead occurs in 4 natural isotopes: 204 pb, 206 pb, 207 pb and 208 pb. the number of electrons in each of lead's shells is [2, 8, 18, 32, 18, 4] and its electron configuration is [xe] 4f 14. Lead-208 Electrons.
From ar.inspiredpencil.com
Lead Atom Electrons Lead-208 Electrons the number of electrons in each of lead's shells is [2, 8, 18, 32, 18, 4] and its electron configuration is [xe] 4f 14 5d 10 6s 2 6p 2. The uranium series (or radium. 208 pb is the most common isotope, having a natural abundance of approximately 52%. 204 pb, 206 pb, 207 pb and 208 pb. . Lead-208 Electrons.
From ar.inspiredpencil.com
Lead Atom Electrons Lead-208 Electrons 204 pb, 206 pb, 207 pb and 208 pb. The uranium series (or radium. 208 pb is the most common isotope, having a natural abundance of approximately 52%. lead occurs in 4 natural isotopes: the number of electrons in each of lead's shells is [2, 8, 18, 32, 18, 4] and its electron configuration is [xe] 4f 14. Lead-208 Electrons.
From material-properties.org
Lead Protons Neutrons Electrons Electron Configuration Lead-208 Electrons the number of electrons in each of lead's shells is [2, 8, 18, 32, 18, 4] and its electron configuration is [xe] 4f 14 5d 10 6s 2 6p 2. 204 pb, 206 pb, 207 pb and 208 pb. 208 pb is the most common isotope, having a natural abundance of approximately 52%. lead occurs in 4 natural. Lead-208 Electrons.
From cabinet.matttroy.net
Lead Periodic Table Protons Neutrons And Electrons Matttroy Lead-208 Electrons 208 pb is the most common isotope, having a natural abundance of approximately 52%. 204 pb, 206 pb, 207 pb and 208 pb. The uranium series (or radium. lead occurs in 4 natural isotopes: the number of electrons in each of lead's shells is [2, 8, 18, 32, 18, 4] and its electron configuration is [xe] 4f 14. Lead-208 Electrons.
From www.istockphoto.com
Electron Configuration Of Lead Illustrations, RoyaltyFree Vector Lead-208 Electrons 204 pb, 206 pb, 207 pb and 208 pb. The uranium series (or radium. lead occurs in 4 natural isotopes: 208 pb is the most common isotope, having a natural abundance of approximately 52%. the number of electrons in each of lead's shells is [2, 8, 18, 32, 18, 4] and its electron configuration is [xe] 4f 14. Lead-208 Electrons.
From cabinet.matttroy.net
Lead Periodic Table Protons Neutrons And Electrons Matttroy Lead-208 Electrons 208 pb is the most common isotope, having a natural abundance of approximately 52%. the number of electrons in each of lead's shells is [2, 8, 18, 32, 18, 4] and its electron configuration is [xe] 4f 14 5d 10 6s 2 6p 2. The uranium series (or radium. lead occurs in 4 natural isotopes: 204 pb, 206. Lead-208 Electrons.
From 3dwarehouse.sketchup.com
Lead 208 Option 4 3D Warehouse Lead-208 Electrons 208 pb is the most common isotope, having a natural abundance of approximately 52%. the number of electrons in each of lead's shells is [2, 8, 18, 32, 18, 4] and its electron configuration is [xe] 4f 14 5d 10 6s 2 6p 2. lead occurs in 4 natural isotopes: 204 pb, 206 pb, 207 pb and 208. Lead-208 Electrons.
From www.researchgate.net
Microscopic cross sections of radiation capture of neutrons by lead208 Lead-208 Electrons 208 pb is the most common isotope, having a natural abundance of approximately 52%. the number of electrons in each of lead's shells is [2, 8, 18, 32, 18, 4] and its electron configuration is [xe] 4f 14 5d 10 6s 2 6p 2. lead occurs in 4 natural isotopes: 204 pb, 206 pb, 207 pb and 208. Lead-208 Electrons.
From www.numerade.com
SOLVED Lead, chemical symbol Pb, has atomic number 82 and can form Pb2 Lead-208 Electrons The uranium series (or radium. 208 pb is the most common isotope, having a natural abundance of approximately 52%. the number of electrons in each of lead's shells is [2, 8, 18, 32, 18, 4] and its electron configuration is [xe] 4f 14 5d 10 6s 2 6p 2. lead occurs in 4 natural isotopes: 204 pb, 206. Lead-208 Electrons.
From www.buyisotope.com
Lead208, Lead208 Isotope, Enriched Lead208, Lead208 Metal Lead-208 Electrons lead occurs in 4 natural isotopes: the number of electrons in each of lead's shells is [2, 8, 18, 32, 18, 4] and its electron configuration is [xe] 4f 14 5d 10 6s 2 6p 2. The uranium series (or radium. 208 pb is the most common isotope, having a natural abundance of approximately 52%. 204 pb, 206. Lead-208 Electrons.
From www.researchgate.net
Parity violating asymmetry (DWBA) in 208 Pb for 1 GeV electrons at 5 Lead-208 Electrons 204 pb, 206 pb, 207 pb and 208 pb. the number of electrons in each of lead's shells is [2, 8, 18, 32, 18, 4] and its electron configuration is [xe] 4f 14 5d 10 6s 2 6p 2. 208 pb is the most common isotope, having a natural abundance of approximately 52%. The uranium series (or radium. . Lead-208 Electrons.
From ar.inspiredpencil.com
Lead Atom Electrons Lead-208 Electrons the number of electrons in each of lead's shells is [2, 8, 18, 32, 18, 4] and its electron configuration is [xe] 4f 14 5d 10 6s 2 6p 2. 204 pb, 206 pb, 207 pb and 208 pb. The uranium series (or radium. lead occurs in 4 natural isotopes: 208 pb is the most common isotope, having. Lead-208 Electrons.
From ar.inspiredpencil.com
Lead Atom Electrons Lead-208 Electrons The uranium series (or radium. 204 pb, 206 pb, 207 pb and 208 pb. 208 pb is the most common isotope, having a natural abundance of approximately 52%. the number of electrons in each of lead's shells is [2, 8, 18, 32, 18, 4] and its electron configuration is [xe] 4f 14 5d 10 6s 2 6p 2. . Lead-208 Electrons.
From www.istockphoto.com
Lead Atom Stock Illustration Download Image Now Lead, Atom, Balance Lead-208 Electrons the number of electrons in each of lead's shells is [2, 8, 18, 32, 18, 4] and its electron configuration is [xe] 4f 14 5d 10 6s 2 6p 2. 208 pb is the most common isotope, having a natural abundance of approximately 52%. lead occurs in 4 natural isotopes: 204 pb, 206 pb, 207 pb and 208. Lead-208 Electrons.
From www.numerade.com
SOLVEDThe most prevalent isotope of lead is ^208 82 2 Pb. a. How many Lead-208 Electrons The uranium series (or radium. lead occurs in 4 natural isotopes: 208 pb is the most common isotope, having a natural abundance of approximately 52%. the number of electrons in each of lead's shells is [2, 8, 18, 32, 18, 4] and its electron configuration is [xe] 4f 14 5d 10 6s 2 6p 2. 204 pb, 206. Lead-208 Electrons.
From ar.inspiredpencil.com
Lead Atom Electrons Lead-208 Electrons lead occurs in 4 natural isotopes: 204 pb, 206 pb, 207 pb and 208 pb. 208 pb is the most common isotope, having a natural abundance of approximately 52%. The uranium series (or radium. the number of electrons in each of lead's shells is [2, 8, 18, 32, 18, 4] and its electron configuration is [xe] 4f 14. Lead-208 Electrons.
From ar.inspiredpencil.com
Lead Atom Electrons Lead-208 Electrons The uranium series (or radium. lead occurs in 4 natural isotopes: the number of electrons in each of lead's shells is [2, 8, 18, 32, 18, 4] and its electron configuration is [xe] 4f 14 5d 10 6s 2 6p 2. 208 pb is the most common isotope, having a natural abundance of approximately 52%. 204 pb, 206. Lead-208 Electrons.